BID relief, with
mucopenetrating power
See how AMPPLIFY® technology works
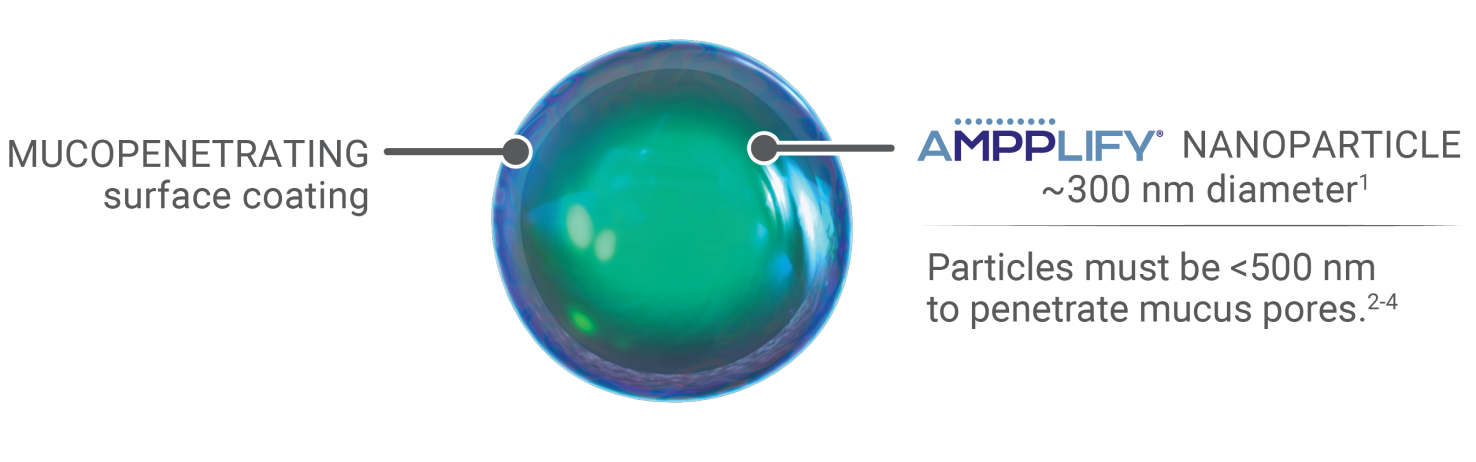
Nanoparticles combined with a mucopenetrating surface coating

• Mucins in the tear film play an important role in protecting the eye from foreign objects. However, they can also limit penetration by traditional suspension eye drops1
• AMPPLIFY® uses nanoparticles of active drug with a proprietary coating to allow the drug to penetrate through the mucus barrier1,5
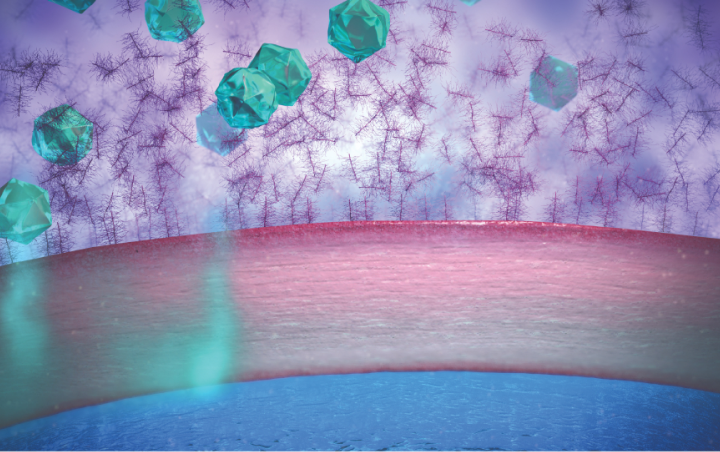

Traditional suspension eye drops adhere to mucins and can be rapidly cleared through blinking1,5
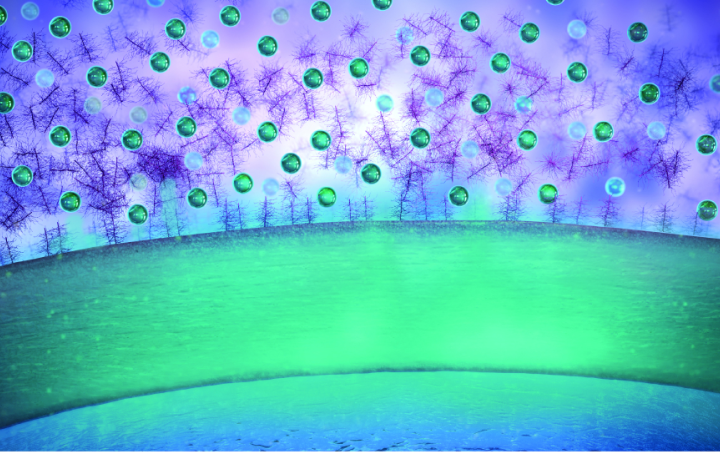

AMPPLIFY® is designed to increase5:
• Ocular surface tissue distribution
• Penetration to the cornea and conjunctiva
REFERENCES
1. Popov A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J Ocul Pharmacol Ther. 2020;36(6):366-375.
2. Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med. 2000;162(5):1905-1911.
3. Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453(1):56-64.
4. Liu M, Zhang J, Shan W, et al. Developments of mucus penetrating nanoparticles. Asian J Pharm Sci. 2015;10(4):275-282.
5. Schopf L, Enlow E, Popov A, Bourassa J, Chen H. Ocular pharmacokinetics of a novel loteprednol etabonate 0.4% ophthalmic formulation. Ophthalmol Ther. 2014;3(1-2):63-72.
Indication
INVELTYS® (loteprednol etabonate ophthalmic suspension) 1% is indicated for the treatment of post-operative inflammation and pain following ocular surgery.
Important Safety Information
Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. If this product is used for 10 days or longer, IOP should be monitored.
Use of corticosteroids may result in posterior subcapsular cataract formation.
Use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids. The initial prescription and renewal of the medication order should be made by a physician only after examination of the patient with the aid of magnification such as slit lamp biomicroscopy and, where appropriate, fluorescein staining.
Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection.
Use of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex).
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local steroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use.
In clinical trials, the most common adverse drug reactions were eye pain (1%) and posterior capsular opacification (1%). These reactions may have been the consequence of the surgical procedure.
INVELTYS® is contraindicated in most viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
Please see full Prescribing Information for INVELTYS®.
